Regulatory Solutions
IT DEVELOPMENT
- System Integration Connectors
- Data Migration System 2 System
- Report Automation (Safety)
- Master Data Management
-
Regulatory Intelligent Reporting
-
Global Registration Tracking
-
Commitment Tracking
SERVICES
- Compliant Submission Packages
- Legacy Data Conversion & Standardization ex. CDISC Conversion
- Aggregate Safety Reporting
- – DSUR
- – PSUR/PBRER
Biometrics
DATA MANAGEMENT
-
Data Management Plan
-
Data Cleaning
BIOSTATISTICS
- Design Consideration
- – Operating Characteristics
- – Sample Size
- Interim Analysis & Adaptation
- Modeling & Simulations
- Statistical Analysis Plan
- Data Analysis
STATISTICAL PROGRAMMING
- Analysis & Reporting Plan
- TLF Production
- Submission Data Sets
- Data Format Conversion & Standardization (ex. CDISC)
Data Analytics
DATA ANALYTICS
- Model Building & Validation
- Advanced Interactive Data Visualization
- Machine Learning and Artificial Intelligence Algorithms
- Report production
CLINICAL TRIAL SIMULATIONS
- Study Design Simulations
- – Design Comparisons
- – Operating Characteristics
- – Incorporation of Enrollment Patterns
- – Sensitivity Analysis and Robustness
- Deviation & Validation of Decision Rules
- Interim Analysis: Rules and Performance
- Sequential and Group Sequential Designs: Implementation and Evaluations
- Adaptive Design (RA, CARA, Population Enrichment, …): Implementation and Evaluations
- Decision Making Framework
SOFTWARE ENGINES
- Development of Software Engine Prototypes
- – RBM & CSM Systems
- – Report Automation
- – Automated Data Analytics
- – Prediction Algorithms
- AI Enabled Computational Platform for High Volume Digital Data
- Medical Device Software
Strategic Consulting
CLINICAL PROGRAM DEVELOPMENT
- Study Design and Protocol Development Consulting for
- – Pharmaceutical and Biotech Industry
- – Medical Device Industry
- – Virtual (site less) Studies
- Portfolio Management
- All Stages of Clinical Studies
- – Pre-Clinical -> Phase 1 -> Phase 2 -> Phase 3 -> Phase 4
- GO / No GO Decisions
- Adaptive Designs
- Optimal Data Analysis Methodology
DATA COLLECTION & MANAGMENT
- Risk Identification and Mitigation Strategies
- Data Quality Control
- Traceability & Security
- Monitoring & Adaptation
- Randomization
ADAPTIVE DESIGN ENABLING PLATFORM
- Design Derivation and its Integration Into the Study
- – Monitoring and Enabling Decision Making
- – Endpoint(s) Monitoring Algorithms
- – Safety Evaluations
- – Prediction of Optimal timing for Interim Analysis
- – Data Integrity Monitoring
- Randomization
- – Ratio Adaptation
- – Complex Stratifications
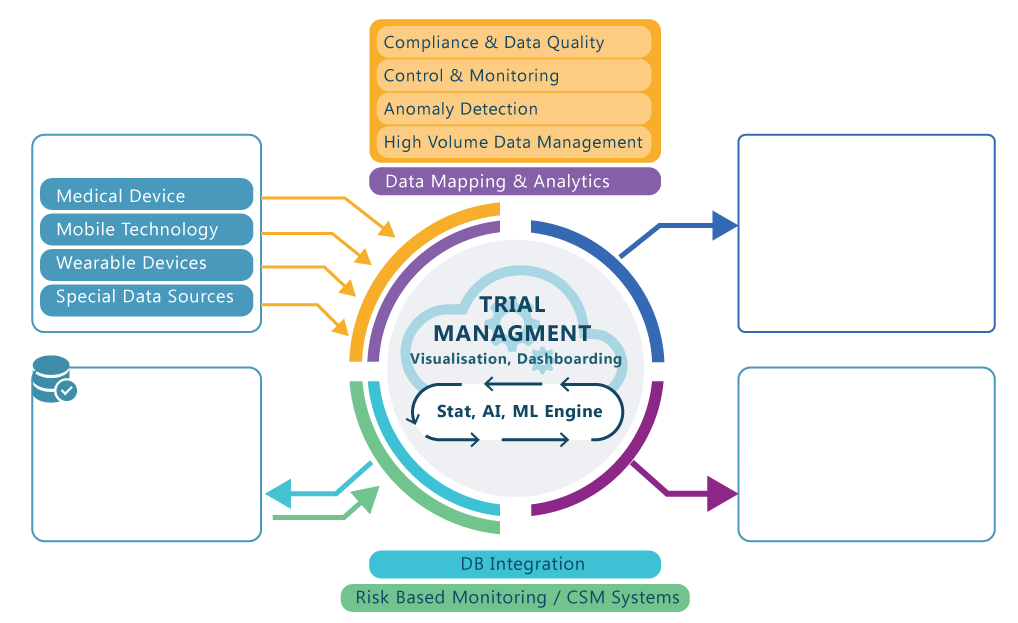
Adaptive Design
ADAPTIVE DESIGN ENABLING PLATFORM
- Endpoint(s) Monitoring Algorithms
- Randomization Ratio Adaptation
- Safety Evaluations
- Interim Analysis Timing
- Data Integrity Monitoring
- Drug Supply Management
CONSULTING ON
- Design Development
- Evaluations & Design Selection
- Operational Challenges
- Regulatory Aspects
- Analysis Methodology
Patient Management
PATIENT MANAGEMENT
- Patient Engagement with eConsent, Patient Engagement, and Clinical Supply Management Modules
- Direct (from Patients) Data Collection with Medical Devices, Apps, EHRs, Diaries, etc.
- Easily Auditable Ledger of Events with Time Tracking
- Enabling Virtual Trials, Patient-Centric Trials, Data Sharing and Collaborative Research
Benefits using ConsilX LifeLedger™
- Improved Operational Framework
- Real Time Data Access From Multiple Sources -> Improved Remote Monitoring and Data Review
- Connecting Stakeholders and Participants (sponsor, investigators, patients and others)
- Sharing of patients data during trial and post trial
- Creating a pool of patients for bettering development timelines
- Secured and permissioned sharing between different R&D groups for the betterment of drug development and health outcomes

