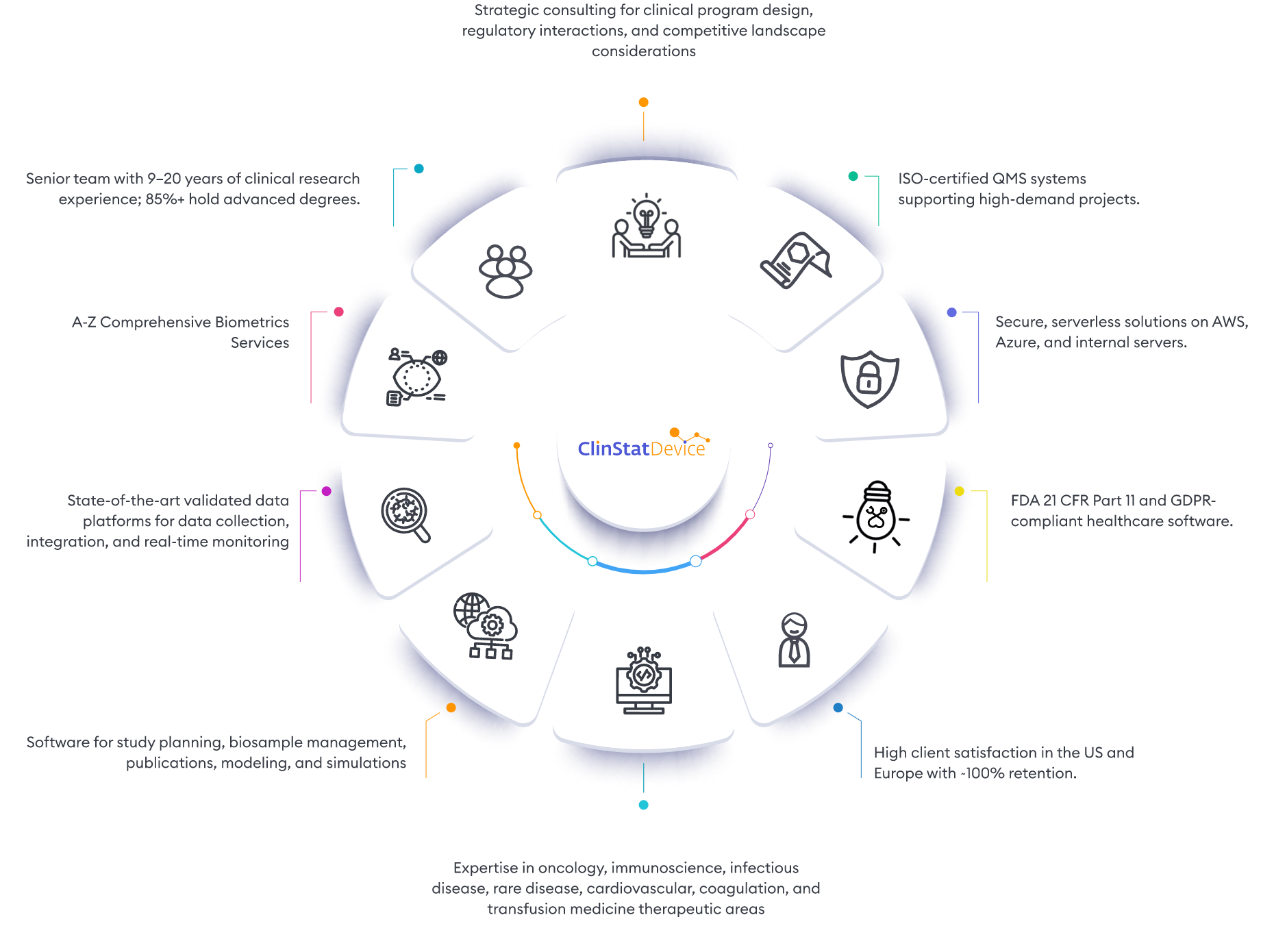
ClinStatDevice (CSD) is your trusted partner in quantitative clinical research solutions. We offer comprehensive support for clinical trial data, including consulting, data analytics, biometrics services, and advanced technology for trial planning, data collection, and real-time monitoring.
Our consulting services provide strategic insights for optimal clinical program strategies, study objectives, and risk mitigation. We have a comprehensive proprietary software suite to cover data collection, trial management and data monitoring. Our specialized software solutions are fully customized for each client’s needs. With a proven track record in complex clinical trials across therapeutic areas like oncology, immunology, infectious diseases, liver diseases, rare diseases, cardiovascular, coagulation, and transfusion medicine, we have achieved numerous successful FDA and EMA submissions in delivery of Phase I–III studies. We look forward to collaborating with you on all design and data aspects of your clinical trials.
Our comprehensive portfolio is showcasing the cutting-edge expertise and diverse spectrum of services offered by CSD. Explore our ground breaking array of successful projects, innovative trials, and impactful studies that epitomize our commitment to advancing healthcare through deep research, advanced methodologies, and unwavering dedication to scientific excellence including classical analysis methods, machine learning & artificial intelligence.
Dive into our portfolio to witness how we’re shaping the future of clinical research and transforming healthcare landscapes worldwide by providing… clinical data collection and reporting system (EDC or patient registry), centralized patient data monitoring, safety and risk based monitoring platforms, bio-sample tracking management and else.


Information Security Management Systems

Quality Management Standard

The Standard for Privacy Information Management System
Address:
269 Main Street #2006 Reading MA, 01867
Email:
info@clinstatdevice.com
Phone:
1-617 480-7727